Test Method Approach
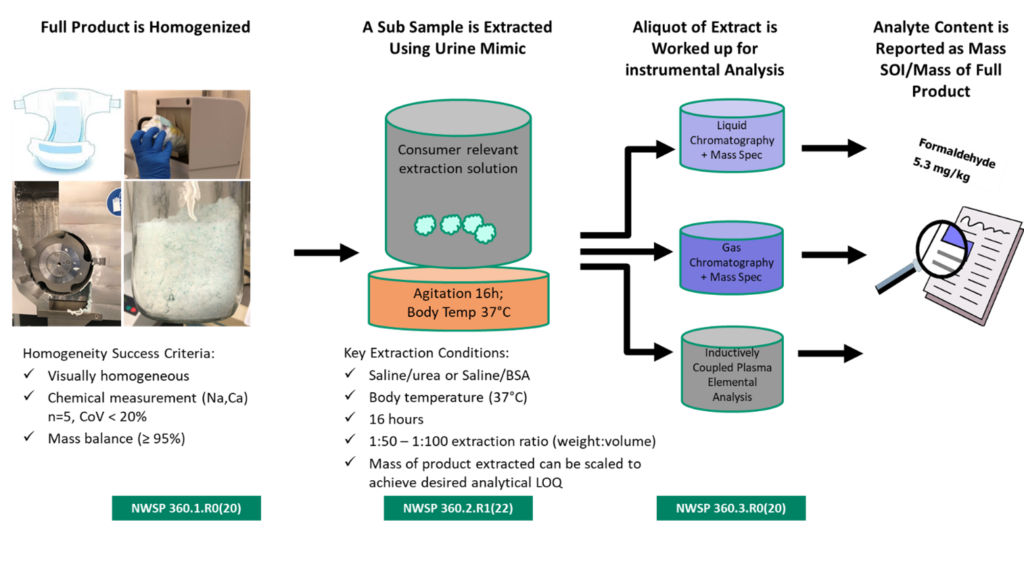
Representation of the Three Test Methods NWSP360.1/2/3
NWSP 360.1 Sample preparation:
A necessary starting point of any overall assessment method is to define some means of bringing a fluid of choice into contact with an article (or portions of an article) to extract possible trace chemicals. A range of approaches were considered. The intent of the sample preparation (as for the analyte extraction) is to capture as many aspects as possible that reflect consumer usage while enabling a procedure that is straightforward, scalable, and robust, and does not introduce factors (e.g. very high pressure) that clearly don’t reflect consumer use.
The EDANA method is based on a milling process, in which the product to be tested is milled in its entirety. All components are taken into account. The homogeneity of milled samples is assessed by measuring the amounts of defined markers in series of sub-samples and the result is a pre-requisite for the further extraction and analytical steps. A milled sample is an over-exaggeration of a full product since it has a larger surface area and thus an extraction method would always extract more from a milled sample. Although the milling process is requirement based, EDANA also provides details of equipment, brand and model, cutting rotor type and speed, duration of the milling per step and the mesh of the sieves used in the outlet of the mill as a practical guidance. Attention is also given to the type of sample containers to avoid unintentional contamination of the sample.
NWSP 360.2 Analyte extraction:
The next step of the overall method is that a specimen of the milled, homogenized sample is extracted in an excess of extraction liquid. Based on the intent to test in a consumer relevant way, scientific experts from the industry decided to use aqueous solutions that mimic urine and menses as the extraction fluid of choice for baby diapers, adult incontinence products and feminine hygiene products. Based on literature review, various potential compositions for these solutions were selected, and experiments were devised to elucidate the differences between them, if any, in terms of extraction performance.
It is important to emphasize that there is no standard “synthetic urine,” because the composition of baby urine itself depends on variables like age and health of the baby, feeding habits and cultural differences. Extraction composition tests revealed that the precise composition of the synthetic urine did not have a significant impact on the results. It was therefore decided to work with a synthetic urine recipe (saline/urea mixture in water) that every laboratory can easily make and that is least influenced by contamination of its component ingredients. This choice contributes to the robustness of the overall method without impacting the extraction performance.
Similar work to identify the optimal composition of menses was concluded in 2022 resulting in the choice for an extraction liquid enriched with proteins (details are provided in NWSP 360.2 R1 (22).
Another range of tests was performed to assess the influence of the key extraction parameters including the incubation time, incubation temperature, type of agitation and the extraction ratio (this is the amount of extraction liquid per gram of dry sample). These tests were all repeated multiple times per combination of parameters to allow statistical evaluation and fact-based decision taking.
NWSP 360.3 Analytical instrumental analysis:
The analytical instrumental analysis for testing trace chemicals in aqueous solutions is based on standard laboratory methods and detection techniques (GC-MS, GC-ECD, HPLC, etc.) that have reached a high level of sophistication. Using these well known, validated and routine analytical techniques in the EDANA procedures contributes to the goal of releasing an AHP testing method that can be executed by a wide range of laboratories throughout the world.
However, these methods often allow a certain degree of variability in their execution in a lab. The EDANA method provides more specific instructions for the reporting of results and potential corrections based on results in blanks. This helps ensure that each laboratory’s work is as comparable as possible to minimise uncertainty and reliably report test results with the correct limit of quantification (LOQ).